A hundred metres below the surface of the Black Sea, life is tough. At that depth, oxygen levels are low and sunlight is practically invisible to the human eye. But despite these adverse conditions, one organism still manages to thrive in this hostile environment. Its secret? An incredible ability to convert small amounts of light into energy.
Scientists are fascinated by the way green sulphur bacteria use hyper-sensitive, antenna-like structures called chlorosomes to efficiently convert the tiny particles of light that reach this part of the sea into chemical energy. And recently a group of Toronto-based researchers has found a way to harness the power of these light-harvesting organelles for an unexpected purpose: to improve our ability to see and treat cancerous tumours hidden deep inside our bodies.
In a study published in Angewandte Chemie (June 2018; first author Dr. Kara Harmatys), a TFRI-funded New Frontiers Program Project Grant team led by Dr. Gang Zheng (Princess Margaret Cancer Centre) explains how they set out to replicate the unique properties of these incredibly efficient light-harvesting organisms in their lab, hoping that they could serve as a contrast agent that would allow doctors to better understand the shape and size of hard-to-reach tumours.
After successfully creating a biomimetic system that copied the molecular structure of the chlorosome organelles that live in the bacteria, the team was faced with a second challenge: making it safe and useful in humans. To do this, they drew inspiration from a high-density lipoprotein nanoparticle that transports fat molecules in human blood, which they managed to replicate in their lab and combine with their newly created biomimetic chlorosome.
This dual-biomimetic system is the first of its kind and has several promising applications: in addition to being a contrast agent that will help researchers see tumours more clearly, it will also play a role in photodynamic therapy (PDT), a promising treatment against cancer that uses light-activated drugs, called photosensitizing agents, to kill cancer cells.
“We envision this agent will improve current surgical procedures by helping surgeons view tumours they are operating on in real-time, while also providing minimally invasive and curable PDT for surgically inaccessible tumours or tumours that are close to anatomical structures,” said Dr. Zheng. “So far we’ve had promising preliminary results and are excited to continue exploring the imaging and phototherapeutic applications of this agent.”
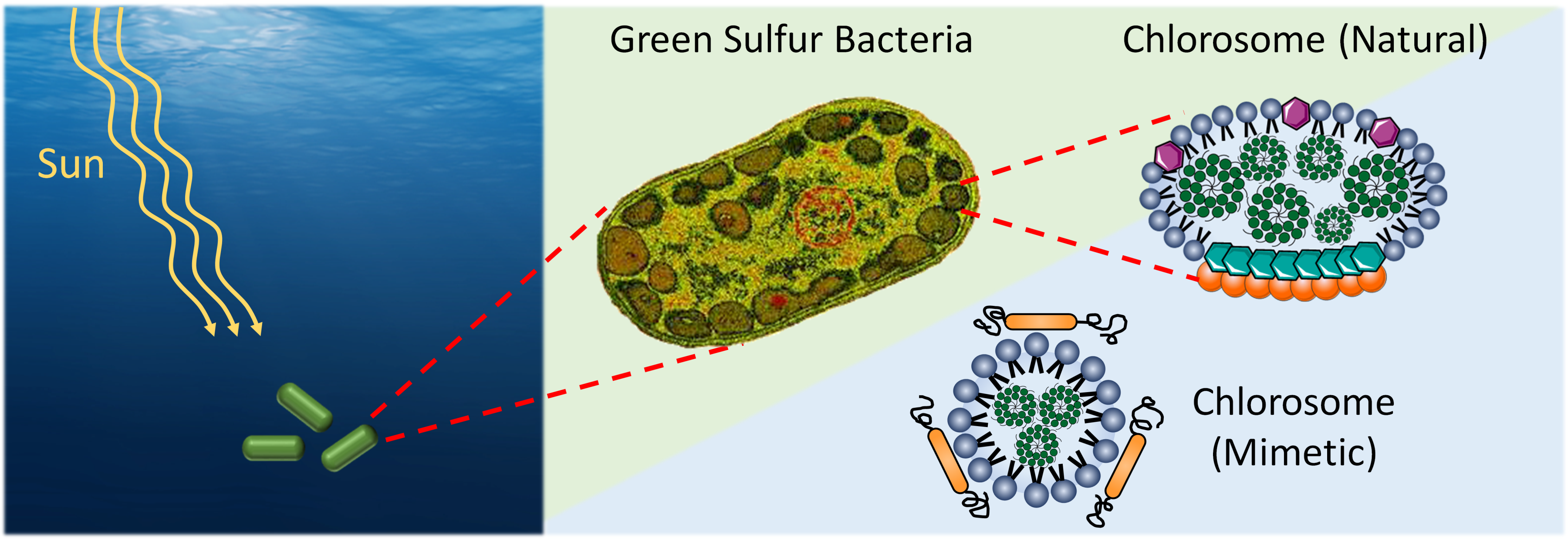
The green sulphur bacteria has the most efficient known light-to-energy conversion ratio in the natural world, which is exactly why Dr. Zheng's team decided to copy its molecular structure to see if it could be used as a contrast agent.
Study
Multipronged Biomimetic Approach to Create Optically Tunable Nanoparticles
Authors
Kara M. Harmatys, Juan Chen, Danielle M. Charron, Christina M. MacLaughlin, and Gang Zheng
Funding
This study is partly funded by a Terry Fox New Frontiers Program Project Grant in Nanoparticle-Enhanced Photoacoustic Imaging for Cancer Localization and Therapeutic Guidance